 AstraZeneca covishield claimed its rare side effect,thrombotic thrombocytopenic syndrome. A critical condition characterized by the formation of blood clots alongside reduced platelet levels.
AstraZeneca covishield claimed its rare side effect,thrombotic thrombocytopenic syndrome. A critical condition characterized by the formation of blood clots alongside reduced platelet levels.
Patient Education and Awareness plays a significant role in curbing down mortalities.
Why was the vaccine launched despite side-effects?
The emergency launch of the vaccine occurred following a phase 2 clinical trial due to a favorable benefit-risk ratio. This decision was made as the benefits of the vaccine outweighed its side effects, resulting in the highest benefit-risk ratio possible.
After two years, is it worth fearing? Thrombosis Risk Factors
“The risk is highest when you get the first dose, but it lowers with the second dose and is lowest with the third. If a side effect has to happen, it will show up within the initial two to three months,” Gangakhedkar told News18.
“There is a need to understand that the risk is close to just 7 to 8 people out of 10 lakhs getting the vaccine,” he added.
Difference between TTS and VITTS?
TTS is a condition that may occur after vaccination, accounting for various potential underlying causes. Can be triggered by various factors, including certain medications, infections, or underlying health conditions.
VITT specifically refers to cases with a clear link to vaccination, particularly certain COVID-19 vaccines, and implies an immune-mediated mechanism.
These cases often have a higher mortality rate and require urgent and specific diagnosis and treatment.
What is TTS syndrome?
Thrombotic Thrombocytopenia Syndrome (TTS), also known as “vaccine-induced immune thrombotic thrombocytopenia (VITT),” is a rare condition that emerges following an immune response to the vaccine.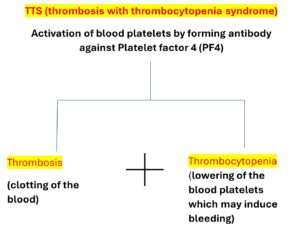
Thrombosis and thrombocytopenia, both cycles happen simultaneously, an autoantibody called platelet factor 4 ,(PF4) antibody, activated the platelets to form clot causing thrombosis, platelet get consumed and thus lowering their number causing thrombocytopenia as a part of the condition.
The blood clots can occur in any part of the body, including:
- The brain (referred to as cerebral venous sinus thrombosis, or CVST)
- The abdomen (known as splanchnic vein thrombosis)
- The lungs (referred to as pulmonary embolism)
- Limb veins (referred to as deep vein thrombosis, or DVT)
- Arteries (known as arterial thrombosis)
Identifying High-Risk Groups for Vaccine-induced Thrombotic Thrombocytopenia (VITT)
Vaccine-induced Thrombotic Thrombocytopenia (VITT) has the potential to affect anyone who receives these vaccines.
Symptoms typically manifest between 5 to 42 days post-injection in healthy individuals.
However, certain groups may face a slightly higher risk, including females and individuals under the age of 60, those with a history of blood clotting disorders, or individuals with specific underlying health conditions.
Females who are on estrogen replacement therapy or oral contraceptives are at particularly high risk.
Additionally, individuals with a history of heparin-induced thrombocytopenia should exercise caution.
Patients who experience severe allergies or immediate reactions to polyethylene glycol (PEG) and its derivatives (e.g., polysorbates) within 4 hours should avoid Pfizer-BioNTech or Moderna mRNA vaccines.
Moreover, certain medical conditions, such as obesity, cardiovascular disease, or diabetes, may elevate the risk of developing blood clots, potentially predisposing individuals to TTS.
Vaccine Mechanisms and TTS Risk: Understanding Variations
TTS is believed to be triggered by the immune system’s response to non-replicating viral vector COVID-19 vaccines, such as AstraZeneca and Janssen adenoviral vaccines.
TTS has not been reported with mRNA vaccines like the Pfizer-BioNTech COVID-19 vaccine (Comirnaty) or other types of vaccines, such as live attenuated or protein subunit vaccines.
mRNA vaccines, such as Pfizer and Moderna, modify mRNA to produce a protein found on the surface of the SARS-CoV-2 virus, triggering an immune response that produces antibodies.
Modified Adenovirus vector-based vaccines utilize a modified vector virus incapable of replication or causing illness, carrying genetic material encoding the spike protein of SARS-CoV-2. Examples include Sputnik V, AstraZeneca (ChAdOx1-S/AZD1222), CanSino, and Janssen (Ad26.COV2).
Live-attenuated and inactivated virus vaccines contain weakened or inactivated pathogenic viruses that induce an immune response. Examples include Bharat Biotech’s Indian vaccine and the Valneva vaccine in development by the European Union.
Subunit vaccines contain specific parts of the pathogen, such as sugars or proteins, triggering an immune response. The Novavax vaccine NVX-CoV2373 has successfully completed Phase III clinical trials.
Understanding Short-Term Side Effects of Covishield
Short term side-effects last for 24-48 hours, which very is normal.
- Injection site tenderness
- Injection site pain
- Fatigue
- Headache
- Malaise
- Myalgia
- Pyrexia (feverishness)
- Fever >38°C
- Flu-like symptoms (joint and muscle pain, headache) that may last for 1-2 days after vaccination.
What are the symptoms of VITT?
Severe long -term symptoms demand urgent treatment.
peak period of such symptoms is 6-14 days after vaccination.
- Chest or back pain
- Breathlessness
- Shoulder swelling or coldness
- Blurred vision
- Extreme and deteriorating headache following vaccination.
- Intermittent bleeding
- Several minor bruising
- Red or purple stains
- Under skin blood blisters
Diagnostic Steps for Suspected VITT: What to Look for and How to Confirm?
Timely interventions can save lives.
If VITT is suspected, the following diagnostic steps should be taken:
A low platelet count is a clear indication for VITT.
- CBC with platelet count and peripheral smear. platelet count < 150,000 per microliter.
Smear is needed to rule out clumping or fragmentation of platelets.
- Imaging thrombosis to detect and visualize blood clots (thrombosis) in the body., focused on detection of cerebral sinus venous thrombosis (CSVT) with CT or MRI venogram, splanchnic thrombosis, pulmonary emboli, and/or DVT.
- D-dimer test: the majority of VITT patients have markedly elevated values, over four times the upper limit of normal.
D-dimer is a protein fragment that is released into the bloodstream when a blood clot breaks down and predicts likelihood of clot.
- Fibrinogen: some VITT patients are reported to have low values. It’s a protein that forms blood clots thus test indicates coagulation status of blood.
Test for thrombocytopenia.
- The PF4-heparin ELISA test- with almost 100 percent of reported cases showing positive assays, detects antibodies against platelet factor 4 (PF4) complexed with the antigen associated with VITTS in the blood.
- It suggests the presence of thrombocytopenia regardless of the test result. However, a negative test result does not exclude the potential for thrombocytopenia in VITTS.
Indications:
Thrombosis is confirmed when there is a decrease in platelet count, an elevated D-dimer test value, either or both tests show abnormal results.
A patient who presents with thrombosis and a normal platelet count post-vaccination may be in an early stage of VITT.
Top Treatment Strategies for Vaccine-Induced Thrombocytopenia
Intravenous immunoglobulin (IVIG) is the first line therapy to elevate platelet count-it modulates the immune response potentially neutralizing the antibodies causing platelet activation.
followed by non-heparin-based anticoagulation therapy -like direct thrombin inhibitors, these do not involve PF4 binding sites.
Steroid Therapy– may help to dampen the vaccine induced immune response, also stabilize blood vessels, and prevent inflammation.
https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia.
Why to exclude Heparin in TTS, mechanism, and rationale?
A strict no to heparin
Vaccine-Induced Thrombotic Thrombocytopenia (VITT), antibodies form against PF4.
This triggers platelet activation, thrombosis, and decreases platelet count through thrombocytopenia.
Heparin has dual mechanism of action, it binds to antithrombin III as well as has binding for PF4.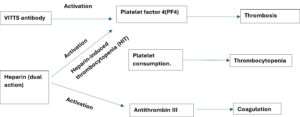
Antithrombin binding cause anticoagulant effect but binding to PF4 trigger an immune response producing antibodies against the complex and causing heparin induced thrombocytopenia leading to platelet activation, thrombosis, increase consumption of platelets and ultimately lowering the count.
Heparin exacerbates this condition by further activating platelets and contributing to thrombocytopenia.
